Abstract
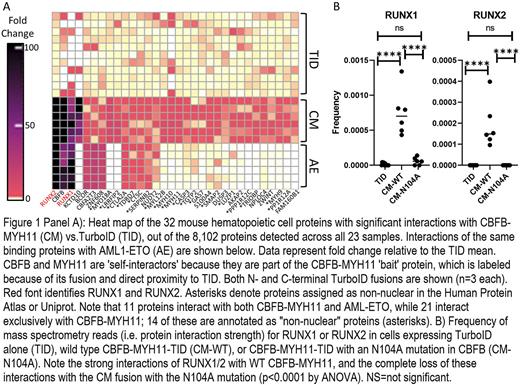
Core Binding Factor (CBF) leukemias are a subset of Acute Myeloid Leukemia (AML) initiated by translocation of genes encoding the heterodimeric core-binding factor (RUNX1 and CBFB) via generation of the oncogenic fusion genes, CBFB-MYH11 and RUNX1-RUNX1T1. While both are classified as favorable risk, relapse occurs in 40-50% of patients with conventional chemotherapy. To better understand the mechanisms by which these fusions initiate AML, we used an MSCV-based, GFP-tagged retroviral vector to introduce a highly active biotin ligase ("TurboID", or "TID") fused to either CBFB-MYH11 ("CM") or RUNX1-RUNX1T1 (AML1-ETO/"AE") at both the N-and C-termini of the proteins into primary murine hematopoietic progenitor cells. With this system, proteins within 10 angstroms of TurboID-fusion proteins are efficiently labeled with biotin, enriched with a streptavidin bead pulldown, and identified using mass spectrometry. We first defined top protein interactors with CBFB-MYH11-TID compared to TurboID protein only ("TID"), and plotted the strength of interactions in the heat map shown in Figure 1A, where each row represents an independent biological replicate (n=11 for TID, n=6 for CM-TID, and n=6 for AE-TID), and each column represents an interacting protein. N- and C-terminal TurboID fusions behaved similarly and are shown together in the figure. AML1-ETO-TID interactions with the same interacting proteins are "passively" plotted, revealing that 11 proteins interacted with both fusions (including RUNX1, RUNX2, and CBFB itself - as expected). AML1-ETO had a unique set of interacting proteins (data not shown) that were primarily nuclear localized, as defined by the Human Protein Atlas (e.g. BCOR, NCOR1, SIN3A, etc.). In contrast, most CBFB-MYH11-interacting proteins were unique for that fusion (including multiple myosin proteins; see Figure 1A), and most were annotated as non-nuclear in the Protein Atlas (denoted with an asterisk in the figure). The predicted cytoplasmic localization of CBFB-MYH11-TID was confirmed by immunofluorescence using an antibody directed against the TID protein. We independently confirmed these results using a CBFB-MYH11-GFP fusion protein, which likewise localized in cytoplasmic aggregates, while a CBFB-GFP fusion was found exclusively in the nucleus. GFP-MYH11 was localized to the cytoplasm, indicating that the MYH11 portion of CBFB-MYH11 directs cytoplasmic localization in a dominant fashion, perhaps by interacting with other cytoplasmic myosin heavy chains. Immunofluorescent staining for endogenous RUNX1 in cells expressing CBFB-MYH11-GFP showed that RUNX1 co-localized with the cytoplasmic CBFB-MYH11, strongly suggesting that the fusion protein acts as a "trap" to sequester RUNX1 in the cytoplasm. To test this hypothesis, we generated a CBFB-MYH11 variant with an N104A mutation in CBFB that is known to disrupt RUNX1 binding. We confirmed complete loss of the CBFB-MYH11 interaction with RUNX1 and RUNX2 using the TurboID system (Figure 1B), and documented restoration of nuclear localization of RUNX1 by immunofluorescence. Using bulk RNA-seq on days 4 and 7 after transduction, we found that WT CBFB-MYH11 overexpression dysregulated many genes (562 up, 352 down, >2-fold change, FDR<0.05), including Runx1 and Runx2, both of which were upregulated. Importantly, the expression phenotype was completely eliminated in cells transduced with the N104A mutated CBFB-MYH11, suggesting that it was dependent on the cytoplasmic sequestration of RUNX1 and/or RUNX2. Finally, analysis of the existing human AML TCGA and Bloodspot transcriptional datasets confirmed upregulation of RUNX1 (p<0.0002 in both datasets) and RUNX2 (p<0.001 in TCGA) in CBFB-MYH11 AMLs relative to non-CBFB-MYH11 AMLs, suggesting that these genes may be activated by a feedback loop that is regulated by functional RUNX1/2 protein levels in the nucleus. In sum, these data suggest that CBFB-MYH11 alters the function of RUNX1 and RUNX2 by sequestering them in the cytoplasm, rather than by directly altering their activity as transcription factors. Thus, novel approaches designed to release mislocalized RUNX1/2 from CBFB-MYH11 cytoplasmic complexes should be explored as a novel therapeutic approach for CBFB-MYH11 AML.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal